How Does The Motion Of The Particles Change When Thermal Energy Is Added?
This focus idea is explored through:
- Contrasting pupil and scientific views
- Critical teaching ideas
- Instruction activities
Contrasting student and scientific views
Student everyday experiences
 At this level, students are expected to 'explain the behaviour and properties of materials in terms of their elective particles and the forces holding them together' (VELS standards Level vi). Notwithstanding, the fact that students may exist able to describe the usual static arrangements of particles in solids, liquids and gases does not mean that they hold a fully particulate view of affair. Enquiry evidence suggests that many students at this age and older still hold a number of alternative conceptions about particles which prove difficult to extinguish. They frequently lack an appreciation of the very small size of particles, attribute macroscopic properties to microscopic particles, have difficulty appreciating the motion of particles in all states of matter and have bug understanding forces betwixt particles.
At this level, students are expected to 'explain the behaviour and properties of materials in terms of their elective particles and the forces holding them together' (VELS standards Level vi). Notwithstanding, the fact that students may exist able to describe the usual static arrangements of particles in solids, liquids and gases does not mean that they hold a fully particulate view of affair. Enquiry evidence suggests that many students at this age and older still hold a number of alternative conceptions about particles which prove difficult to extinguish. They frequently lack an appreciation of the very small size of particles, attribute macroscopic properties to microscopic particles, have difficulty appreciating the motion of particles in all states of matter and have bug understanding forces betwixt particles.
Research: Driver (1987)
Many students who appreciate that matter is particulate still retain some one-time views and consider that particles can change their class (solid to liquid), explode, fire, aggrandize, change shape and color or shrink. Students visualise atoms, molecules and ions to exist piffling brawl-like objects (perhaps because of the way the data has been presented) and this contributes to them confusing the properties of the particles with the macroscopic nature of the materials that they make upwards.
Research: Happs (1980)
These ideas are besides explored in the focus thought Macroscopic and microscopic backdrop.
Students frequently fail to empathize the dynamic nature of particles; they tend to think of them as static. Students may believe that gas particles are moving slowly in ways similar to what they observe when they meet suspended dust particles in a axle of lite. Random particle motion in liquids and gases is a difficult concept for students to appreciate. When asked, "Why don't gas particles fall to the bottom of a vessel?" merely about 50% of students thought that the particles were in constant motility. Students stated that particles were forced apart (by heat interim as a substance) when gases were heated. When gases condensed to a liquid, many students attributed this to increased bonny forces between particles.
Inquiry: Novick & Nussbaum (1981)
Students frequently find it hard to appreciate particle movement in solids and this leads to different conceptions about freezing and melting. Some examples of students' thinking virtually the behaviour of particles in a melting ice block are:
Educatee 1: "The particles start to suspension abroad from each other because of the rise in temperature. When they have broken away from each other, they turn from a crystal form to a solution form."
Student 2: "When a cake of ice is taken out of a freezer, the sudden change of temperature reacts on the particles making them decrease in size."
Scientific view
Atoms are incredibly modest and cannot be seen with fifty-fifty the most powerful light microscope. We utilize multiple models of atoms to assist explain chemic processes and describe their behaviour.
In gases the particles move chop-chop in all directions, frequently colliding with each other and the side of the container. With an increase in temperature, the particles gain kinetic energy and movement faster. The actual average speed of the particles depends on their mass as well as the temperature – heavier particles movement more slowly than lighter ones at the same temperature. The oxygen and nitrogen molecules in air at normal room temperature are moving rapidly at betwixt 300 to 400 metres per second. Unlike collisions between macroscopic objects, collisions between particles are perfectly rubberband with no loss of kinetic energy. This is very different to near other collisions where some kinetic energy is transformed into other forms such every bit rut and audio. It is the perfectly rubberband nature of the collisions that enables the gas particles to continue rebounding after each collision with no loss of speed. Particles are all the same discipline to gravity and hit the bottom of a container with greater force than the superlative, thus giving gases weight. If the vertical motion of gas molecules did not slow under gravity, the atmosphere would take long since escaped from the Earth.
In liquids, particles are quite shut together and move with random motion throughout the container. Particles movement speedily in all directions but collide with each other more frequently than in gases due to shorter distances between particles. With an increase in temperature, the particles move faster as they gain kinetic free energy, resulting in increased standoff rates and an increased charge per unit of improvidence.
In a solid, the particles pack together as tightly as possible in a bang-up and ordered arrangement. The particles are held together too strongly to allow movement from place to place but the particles do vibrate about their position in the structure. With an increase in temperature, the particles proceeds kinetic energy and vibrate faster and more than strongly.
The attractive forcefulness in solids need not be stronger than in liquids or gases. For example the forces betwixt solid helium particles (at -270 degrees C) are still very weak. By comparing, the forces betwixt atomic number 26 vapour particles (requires very high temperatures) are very strong. If you compare different substances that are at the aforementioned temperature, and then the average kinetic free energy of the particles will be the same (i.e. if the particles take the aforementioned mass then they will move with the same speed), but the attractive forces in solids will be greater than those in liquids, which will be greater than those in gases. Attractive forces don't get weaker when a substance moves from the solid to the liquid to the gas state, rather the kinetic energy of the particles increases (implying faster motility), allowing them to overcome the attractive forces.
Critical didactics ideas
- All affair is fabricated up of atoms which are far too minor to see even with the most powerful low-cal microscopes.
- Particles in all states of thing are in abiding motion and this is very rapid at room temperature. A rise in temperature increases the kinetic energy and speed of particles; it does non weaken the forces between them.
- The particles in solids vibrate nearly fixed positions; fifty-fifty at very low temperatures.
- Individual particles in liquids and gases have no stock-still positions and move chaotically.
- The collisions between particles differ from collisions between macroscopic objects in that they are perfectly elastic: i.east. the kinetic free energy of the particles remains abiding and no free energy is transformed into other forms during collisions.
![]() Explore the relationships between ideas nearly movement of particles in the Concept Development Maps - (Chemic Reactions, States of Matter)
Explore the relationships between ideas nearly movement of particles in the Concept Development Maps - (Chemic Reactions, States of Matter)
Students at this level have been exposed to ideas almost particles (including atoms, ions and molecules) on a number of occasions, withal many of them retain alternative or naïve views nigh the nature of particles and these can inhibit their agreement. Aim to prefer teaching strategies that promote dissatisfaction in students with their existing ideas, and promote a scientific conception that is plausible, consistent and useful in a variety of situations.
Teaching activities
Bring out students' existing ideas
It is important to define the majority of students' prior views at the showtime of teaching to establish their existing agreement of the particle model of matter.
Ask students for their ideas about the size of atoms compared with other small things such as cells, bacteria and viruses. This tin exist washed by request them to describe the relative size of these on the aforementioned scale (a scale where a human cell is the size of a page or poster). Bring out the thought that atoms are then much smaller once more. Look for other activities that can help reinforce the idea that particles are very, very small.
Evidence students the conventional drawings of particles in solids, liquids and gases and inquire them if and how fast they think they are moving.
Challenge some existing ideas
A number of the bug raised in the focus idea 'Conservation of mass' are relevant hither and the weighing of a flask containing a pocket-sized amount of acetone before and after evaporation can be used to challenge students' ideas about matter being lighter in the gas state and to raise problems with the static drawings of gas particles in texts. For more than information see: Conservation of mass.
Help students work out some of the 'scientific' explanation for themselves
With a little encouragement, a class tin unremarkably work out by discussion that the particles in gases must exist hitting the bottom of the flask harder than the height and hence that they are afflicted past gravity. This tin be extended to explaining why the Earth's atmosphere thins and eventually ends – the upwardly vertical movement of the particles ceases.
Promote reflection on and clarification of existing ideas and encourage students to identify phenomena not explained by the (currently presented) scientific model or idea
As particles cannot be directly observed, much of the teaching involves looking for credible issues or inadequacies with the sorts of static pictures of particles given in before years. Encourage students to identify these and talk through possible explanations. Some prompts:
- What holds air particles upwards?
- Are air particles moving faster on a windy day?
- How can gases have weight?
- Why don't air molecules fly off into outer space?
If needed, raise bug such every bit these, which will open up upwardly discussion, but it is better if the students themselves come upward with some. Annotation that many of the issues are to practise with gases – information technology is their backdrop that we virtually need a particulate model to explain.
To reinforce the notion of elastic collisions, ask what would happen if collisions between gas particles were non elastic. What practical consequences would there be for people? This can be introduced by dropping dissimilar types of balls (such as a soccer ball, a table tennis ball and a bouncy ball (from toy shops)) and explaining that a boisterous ball behaves more like gas particles.
Open up discussion via a shared experience
Using activities like POE (Predict-Discover-Explain) can assistance students call up about and so question their existing ideas. The following action will help students consider their ideas virtually the movement of particles.
Set up ii pairs of flasks each continued past a valve (run across diagrams below). Both pairs accept brown nitrogen dioxide in the left hand side flask.
| POE (Predict-Find-Explicate) experiments | |
|---|---|
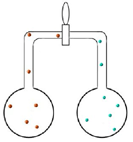 | The commencement pair too has air in the correct hand side flask. Students are asked to predict what will happen when the valve betwixt the two flasks is opened. The brown colour will spread very slowly from 1 flask to the other considering the particles accept frequent collisions with the air particles. |
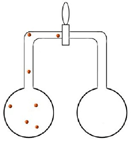 | The second pair of flasks has brown gas in the left paw side flask but the correct paw side flask is completely evacuated. Students are asked again to predict what happens when the valve is opened. The very fast speed of the molecules means that they fill the evacuated flask very chop-chop. |
 Improvidence experiments can reinforce the idea of motility of particles. These tin can also be used equally POEs.
Improvidence experiments can reinforce the idea of motility of particles. These tin can also be used equally POEs.
For case:
- a crystal of copper sulphate is placed in agar gel; the blueish color slowly diffuses through the gel
- a potassium permanganate crystal is placed in a glass and water is slowly added. Come across the epitome. Alternatively water is very slowly added to a solution of potassium permanganate in a burette.
Brownian motion can also exist observed using stereo microscopes when sulphur powder or camphor is sprinkled on the surface of water or ethanol.
Practise using and build the perceived usefulness of a scientific model or idea
A cotton fiber wool piece soaked in ammonia is placed at one end of a long glass tube with another soaked in hydrochloric acid (HCl) placed at the other terminate. Eventually a white ring will course where the two gases run into. The two gases are at the same temperature and thus the particles have the same kinetic energy; the ring forms closer to the source of heavier and thus slower moving HCl. This is predicted by a comparing of the relative molecular masses. Including a strip of universal indicator newspaper in the tube allows the gas diffusion to be tracked. This is an case of a POE where it is useful to describe students' attending to a relevant piece of scientific discipline before they brand their prediction as it builds usefulness for the concept of relative molecular mass (Mr values).
Students need to exist given the opportunity to use the scientific conceptions about particle theory in other settings. Ask students to find and and so explain the changes in terms of particle movement in scenarios such as melting wax or plastic, mothballs (naphthalene) vanishing in a cupboard and the scent of perfume spreading through a room.
Source: https://www.education.vic.gov.au/school/teachers/teachingresources/discipline/science/continuum/Pages/particles.aspx
Posted by: neubauersoman1985.blogspot.com

0 Response to "How Does The Motion Of The Particles Change When Thermal Energy Is Added?"
Post a Comment